The physics of Fire
Some areas are simplified or may be theoretical but will contain the information pertinent to Fires in High Rises. Discussed are some simplified physics and there interpretation in the high rise environment
Fire, or more correctly, combustion, is a chemical reaction. It is usually Exothermic, which means it gives out heat energy. In the context of this article, fires (with conventional fuel sources) may best be described as the rapid oxidation of a combustible material releasing heat, light, and various reaction products such as carbon dioxide and water.
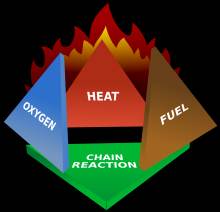
Looking closer at this definition there are 4 requirements for Fire: Heat, Fuel, Oxygen and an ongoing Chain reaction. Each of these requirements must exist in sufficient quantity for the Fire to start and be maintained.
HEAT
Heat is a form of energy; more specifically it is Energy transfer. Heat has a tendency to move from a high-temperature region to a low-temperature region. This process may be carried out by means of conduction, convection and radiation. Conduction and convection require a material to transfer through. For conduction it is a solid, for convection, this will be a liquid or gas. Radiation requires no medium and is a form of electromagnetic radiation.
The study of Heat Energy is a deep and complex area of physics with branches in Thermodynamics, Quantum mechanics and Kinetics.
The generic SI unit for measuring Heat energy is the Joule. In relation to Fire fighting, other systems of measurement are important. Temperature and Heat Release Rate being the most relevant.
4.184 Joules (in terms of heat energy) are required to raise 1 Gram of water by one degree C. The Joule is symbolised with the letter J. Therefore a Kilojoules (KJ) being 1000J and a Mega Joule (MJ) being 1000000J
Heat Release Rates (HHR)are normally defined in Watts and is a measure of the rate at which energy is being used/released in the process of combustion. It may be defined as doing one Joule’s worth of work in one second It is symbolised with the letter W and 1W = 1Js-1 Therefore a Kilowatt (KW) being 1000W and a Megawatt(MWJ) being 1000000W. HHR is one of the most important factors in understanding the hazard’s associated with high rise fire fighting (1)
Temperature is a comparative measurement in Degrees Celsius. It is symbolises with the letter C. At normal pressure water freeze at 0 deg C and boils at 100 deg C
As you can see all these systems of measurement are interrelated so for example 1 kilowatt hour = 3.6 MJ
The application of a heat source, to a fuel in normal air is usually termed the “Source of ignition”. This may range from deliberately set fires where matches and lighters are the commonest source up to a wide range of accidental causes, such as unattended cooking, electrical overload faults and carelessly discarded cigarettes. Once the fire has started, and providing fuel and oxygen are available fires generate their own heat and become self-maintaining.
The actual process of burning (or Oxidisation) involves the chemical decomposition of Organic compounds. Most items in every day domestic use are primarily Organic compounds, for example, Plastics, Wood, fabric, carpet, Cooking oil. The formal name for this decomposition is Pyrolysis.
In pyrolysis it is important to realise that Solids do not burn directly, but that the external heat source or radiated heat from existing combustion causes fuel solids to initially decompose. In many substances the decomposition will be an evolution from solid to liquid to gas or vapour. Some substances will turn directly to gas under exposure to sufficient heat energy . It is this vapour that is the true fuel.
FUEL
All fuel, by definition, possess potential energy. During complete combustion, fuel will be decomposed by heat in the presence of oxygen to ultimately form non-combustible by-products. Fuels may be analysed and measured to quantify how much potential energy would be releases if it was completely combusted. This is referred to as its Calorific value. For example a ton of TNT possesses 4180MJ of potential energy. Black Coal possesses 32500MJ per ton. It can be seen that coal has over 7 times more potential energy if burnt. What is important here is that when TNT decomposes (explodes) it releases all of that energy in a fraction of a second, whereas a ton of lumped coal may burn for many hours. The rate which a fuel burns is dependant on many factors. An important factor is access to oxygen. In most fires fuel is not burnt efficiently resulting in flammable or even explosive gasses being produced.
OXYGEN
Oxygen is an element that exists at normal temperatures and pressures as a colourless, odourless gas with molecular formula O2. It is a component part of Air that is composed of approximately 21% Oxygen and 79% Nitrogen. Most normal combustion requires some level of 02. In general fire fighting term it is accepted that combustion rates decline appreciably as O2 levels start to fall. This is how smothering works. Increasing the O2 level will accelerate combustion (heat energy release). Increasing the availability of O2 is effectively happening when a strong wind ventilates a fire. The fire burns more fiercely because the fuel is exposed to a higher partial pressure of oxygen. Partial pressure refers to quantity of a gas in a volume. The strong wing raises the pressure against the surface of the solid increasing available oxygen.
Some materials that are capable of combustion contain there own oxygen content (for example Thermites) and do not use Oxygen from the Air.
Chemical (Chain) reaction
The chemical reaction in a flame is regarded as a chain reaction. This is where the product or by-product of the reaction causes additional reactions to take place, becoming self promulgating.
The chemical processes occurring in the flame are very complex and typically involve a large number of chemical reactions and intermediate species, most of them radicals. These radicals are atoms or fragments of molecules that are extremely reactive.
Smoke (airborne products of combustion)

Smoke is a collection of tiny solid, liquid and gas particles. Although smoke can contain hundreds of different chemicals and fumes, visible smoke is mostly carbon (soot), tar, oils and ash.
Smoke occurs when there is incomplete combustion (not enough oxygen to burn the fuel completely). In complete combustion, everything is burned, producing just water and carbon dioxide. When incomplete combustion occurs, not everything is burned. Smoke is a collection of these tiny unburned particles. Each particle is too small to see with your eyes, but when they come together, you see them as smoke.
When considered as part of a fire fighting problem, smoke poses two primary problems
It is a fuel :
Various combinations of Pyrolysis (usually Hydrocarbons) and Carbon Monoxide
It is very toxic (Primarily due to HCN- Hydrogen Cyanide and CO-Carbon Monoxide )
A hydrogen cyanide concentration of 300 mg/m3 in air will kill a human within about 10 minutes. A hydrogen cyanide concentration of 3500 ppm (about 3200 mg/m3) will kill a human in about 1 minute ( gaseous hydrogen cyanide is approximately 5% lighter than air)
Carbon monoxide poisoning is the most common type of fatal air poisoning in many countries.[19] Carbon monoxide is colourless, odorless, and tasteless, but highly toxic. It combines with hemoglobin to produce carboxyhemoglobin, which is ineffective for delivering oxygen to bodily tissues. Concentrations as low as 667 ppm may cause up to 50% of the body's hemoglobin to convert to carboxyhemoglobin.[20] A level of 50% carboxyhemoglobin may result in seizure, coma, and fatality (it is about 3% lighter than air)
Due to thermal agitation during a fire, all the products of combustion mix together and act as a single mass. This mass may be lighter than air due to gravity currents (hot air rising)
Air and Smoke movement
Smoke will and can move in unpredictable ways. Most firefighters, in there day-to day fire fighting activity will observe smoke traveling upwards. In the high rise environment ensure that you understand that smoke can and will travel in ANY direction
Air and Smoke movement covers two issues:
1, The ventilation of the fire
2. The tenability of the internal structure (compartmentation)
One of the most complex aspects of high rise buildings is modelling or predicting air flow and smoke movement during a fire. Many different influencing attributed will come into play, not necessarily when expected and not always under the control of the fire-fighters attending.
The Theory of Gases (air) movement
This area of physics is called fluid mechanics. The behaviors of fluids and gases are very similar at a theoretical level.
There exists a set of rules, descriptions and principals that can explain and govern, the behavior, movement and characteristics of all gases (and liquids).
Some of those that are interest are:
Density
The Ideal gas laws (Charles and Boyles law)
The Coandă effect
Bernoulli's principle
Aerodynamics
Density:
The density of a gas will affect its buoyancy. All objects that posse’s density are acted upon by gravitational forces. The overriding gravitational force we experience in our day to day lives is that of the earth.
The most basic observable effect that gravity has in relation to High rise incidents is the movement of heated gasses. It is a well understood principal that Hot air rises. Simplistically, hot air rises because it occupies a greater volume per unit mass of air than cooler air This means it is LESS dense..
To correctly define this action it is better to say that Cool air falls. Cool air is denser, so there is a greater gravitational force acting on it. What is interesting is that there is very little mixing or dilution as cold gasses pass through hot gasses. There are also many other complex physical and mechanical (Kinetic energy & Thermodynamic) effects that contribute to the concept of “Hot air rising” which is more normally called CONVECTION
Ideal Gas laws
|
Robert Boyle |
Jacques Charles |
 |
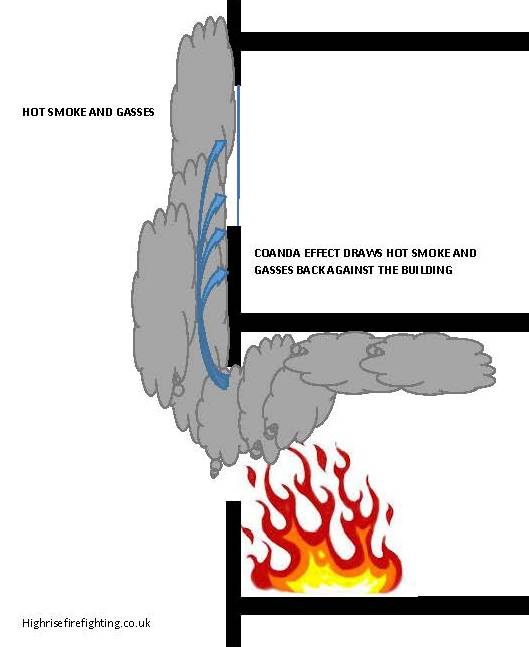
The Coandă effect
To demonstrate , a spinning ping pong ball is held in a diagonal stream of air by the Coandă Effect. The ball "sticks" to the lower side of the air stream, which stops the ball from falling down. The jet as a whole keeps the ball some distance from the jet exhaust, and gravity prevents it from being blown away.
|
| The coanda effect will influence hot gasses escaping from compartments involved in fire. the combined effects of Convection, fire compartment pressurisation or the effect of wind will cause smoke and hot gasses to be expelled from an opening, usually traveling vertically upwards.. The Coanda effect will encourage these products of combustion to be drawn back to the face of the building. This is a common cause of external fire spread. |
|
Vertical spread (Autoexposure) Click to image to enlarge |
Stack Effect
The stack effect is sometimes called the Chimney effect
This is an air current or movement caused by the displacement of air due to its buoyancy. This buoyancy is primarily caused by heat. Typically “hot air rises cold air sinks”. Occasionally this can be caused by altering the chemical makeup of the gasses in the air. In tall columns (stairwells and lift shafts) strong convection currents can form where air within the shaft, comes into contact with air outside the shaft (usually from outside the building). It is important to note that These air currents can travel upwards and downwards
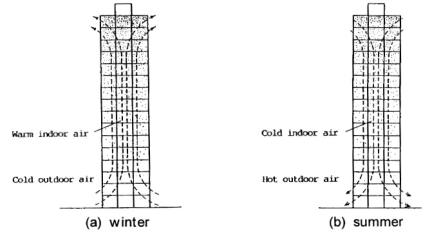
During a cold winter’s night, indoor temperature is higher than outdoor temperature so warmer air in building then rises up. The upward air movement produces negative indoor pressure at the bottom of the building and positive indoor pressure is created on the top. Warmer air flows out of the building near the top and this air is replaces by colder outside air that enters the building near its base. During hot summers day, the reverse occurs when indoor temperature is lower than outdoor temperature.
The stack effect is well researched both in terms of building design for ventilation and the effect it has on smoke travel.
Its process can be computer modelled and the change in pressure mathematical predicted using:
Stack Effect ΔP=Cah(1/T0 – 1/T1)
where: |
|
ΔP |
= available pressure difference, in Pa |
C |
= 0.0342 |
a |
= atmospheric pressure, in Pa |
h |
= height or distance, in m |
To |
= absolute outside temperature, in K |
Ti |
= absolute inside temperature, in K |
On its own it is able to generate considerable pressure differences and resultant air flows. When heated products of combustion enter a shaft the stack effect can produce serious and detrimental air flows.
Wind Pressure.
The strongest Gust of wind ever recorded on earth was 231MPH. In the UK, during the “Great storm” of 1987 the strongest gust was 138MPH
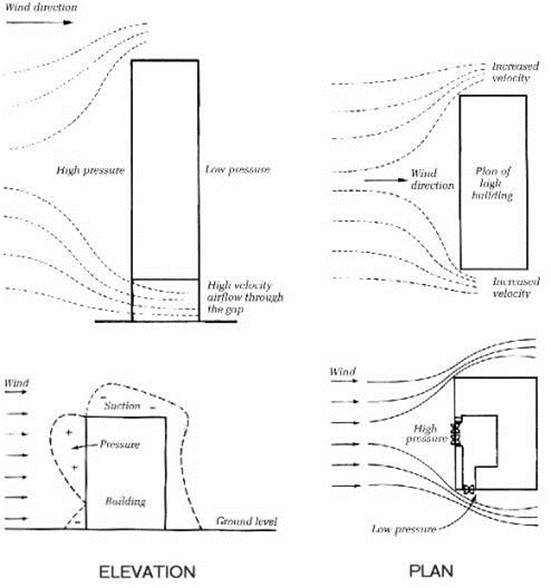
The wind has many effects on High rise buildings (not least causing them to sway or resonate). Wind loading is a very important consideration in the design of tall buildings. The design of the outer surfaces of the building will also affect how air travels over and around a building. Wind speed is nearly always faster at the upper stories of a tall building. Nearby buildings will also have a marked affect on local wind currents.
Another variable aspect of the wind is the direction it blows in. For every face of the building, a prevailing wind will affect it in different way, causing areas of high and low pressure. These pressure differentials will affect air flow inside the building if there are any openings. (Windows doors etc..)
Heating, Ventilation and Air Conditioning (HVAC) Systems
Modern High Rise accommodation buildings may be serviced by HVAC. Most HVAC is carried out using air ducting, usually recycling (and either heating or cooling) the air in the building. It is normally mixed (refreshed) with a quantity of air from outside the building.
These systems are able to create very slightly positive pressure in buildings, causing air flow and air currents to travel further than would normally be expected. Because of the recycling aspect of HVAC systems, most are built with smoke dampers to stop smoke being “blown” around a building. Some have Automatic smoke detection in the ducting system that if it activates, turns off the HAVC systems.
The Piston Effect
The piston effect is created by the travel of lift cars in lift shafts. It is discussed in the section on lifts. In single shaft lifts its effect can be two fold. It can create Positive or negative pressure in lift lobbies and it can draw smoke into (protected) lift lobbies and ultimately into lift shafts, making them unusable. The piston effect can be greatly increased in High Speed lifts.
The piston effect can be exaggerated in shafts containing more than one lift car.
Pressurisation systems (for Fire fighting)
Engineered solutions (covered by BS5588 Pt 4 and Pt5) and Approval Document B for pressurised stairwells, Lift shafts, escape routes and protected lobbies may be found in some residential High Rise buildings. They are designed to restrict smoke movement and help proved occupants with safe, clear escape routes and to assist the ingress of fire fighters.
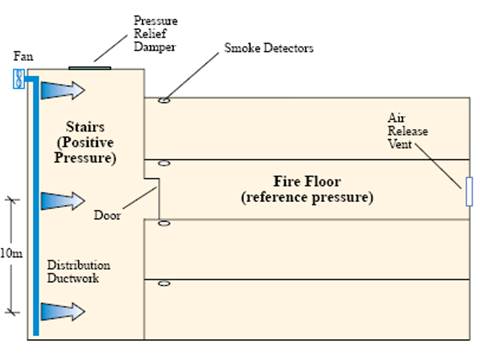
Pressurisation systems can be of great assistance to the Fire service in creating tenable working conditions in lobbies and stairwells close to the fire compartments. But they are mechanical systems that are able to fail and their effect is greatly reduces when doors or windows are opened/breached (either deliberately or as a result of fire spread). Some research is being carried out to investigate whether it would be beneficial to pressurise lift shafts for Fire Fighting operations.
Mobile Positive Pressure Ventilation PPV (PPA)
Although PPV has been discussed since the early 50, its only since the 80’s that UK Fire Services have started to look at its tactical use. Still today many have not adopted any form of powered PPV
PPV is a system that Fire services can use to mechanically create a positive pressure (an ensuing) air flow into a building allowing products of combustion (smoke and heat) be be directed to a known, controlled vent point. One of the biggest drawbacks of using PPV at high rise fires is difficulty in the safe creation and control of the Venting point.
Its Use in High rise buildings is complex and involves close coordination and control. It may well require a number of fans top create the required pressurisation levels. Recent American and Canadian research has produced some impressive results at live fire trials , but this was carried out with experienced ventilation crews, sometimes using very large “Truck mounted” fans. The experience and expertise to use there safely and reliably in high rise fires (especially in any offensive mode) in the UK is very limited.
Mechanical Automatic opening window and roof Vents
As Part of BS and Approval Document B, High rise building staircases or Lobbies are usually designed to have some form of automatically operating vent system.
They are normally activated by the Automatic Fire alarm system in the building. These are to create tenable conditions in escape routes during evacuation of the building. Often they will open, but are not overrideable or even closable. The exact specification for how they should operate is open to interpretation and “engineered solutions” The author has seen some recent (modern) examples of these and although they are compliant with building regulations, they will almost certainly have a detrimental affect on smoke travel in the building. Like most Fire-fighters , The author prefers to initiate and control Ventilate when he feels is appropriate, not when an automatic system chooses.
Fire Fighters Shaft
Is a design requirement under BS 5588 Part 5, These are shafts that are naturally ventilated but Automatically controlled. Upon Smoke/Fire detection, a normally roof mounted vent at the top of the shaft will open together with a damper vent on the floor(s) affected. The shaft is usually one long communal shaft running the length of the building and is nominally 1.5m square

Reference’s
1 Heat release Rates (NIST/ Fire Safety Journal 1994) Http://fire.nist.gov/bfrlpubs/fire92/PDF/f92019.pdf
Adapted from Source :
MF2012